C.1 Thermodynamics in Earth’s Systems
How can we slow the flow of energy on Earth to protect vulnerable coastal communities?

Unit Summary
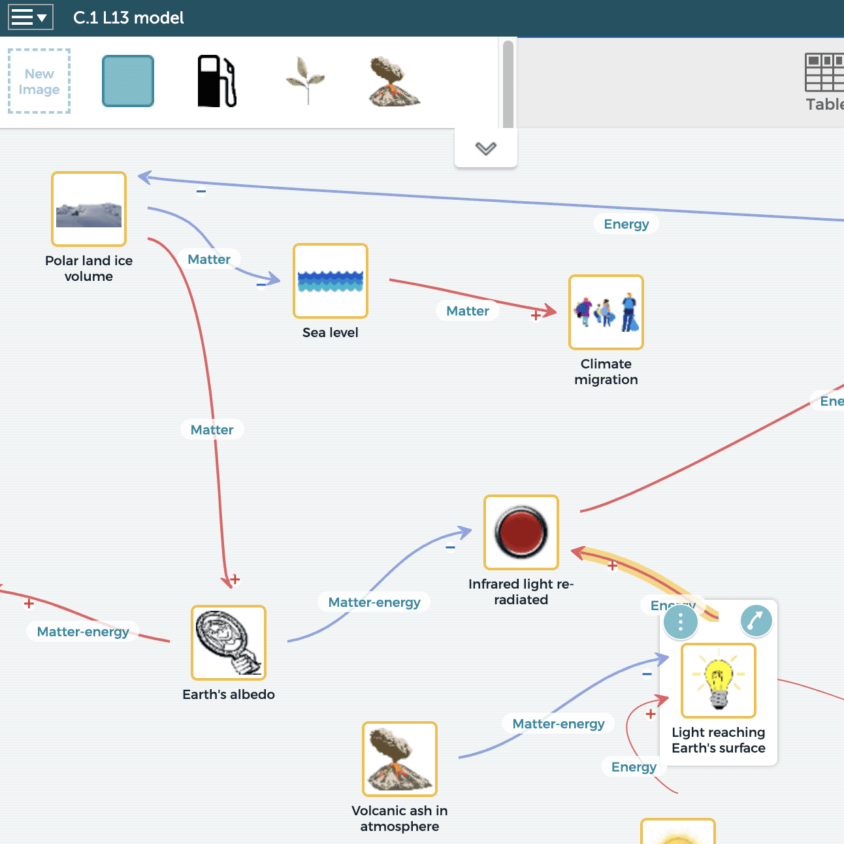
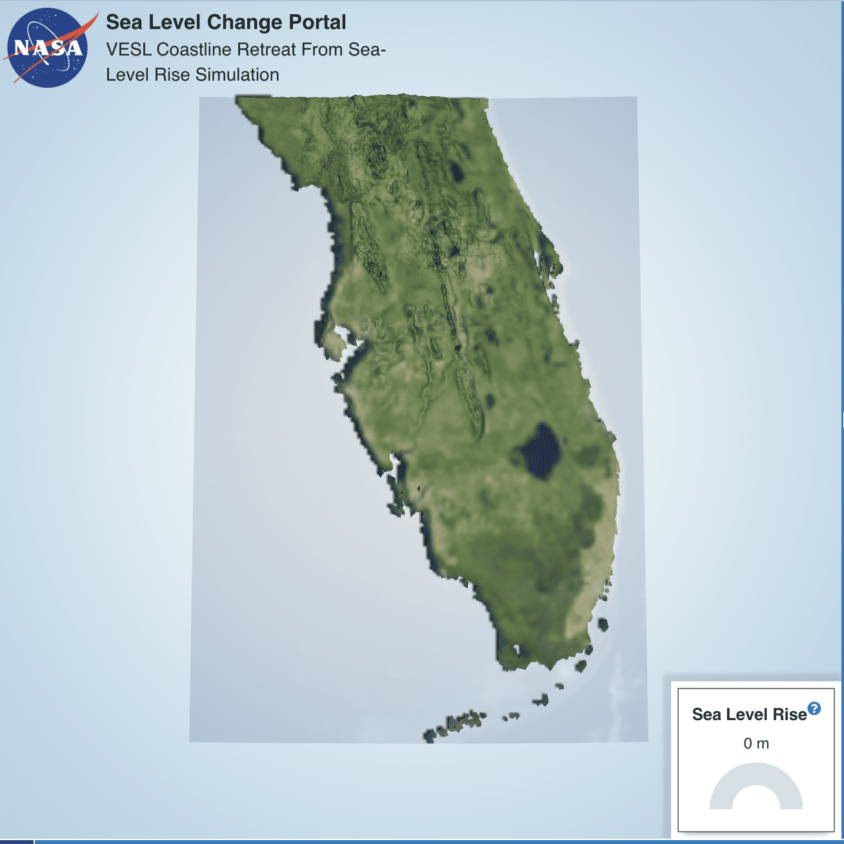
Why is the sea level rising, causing some people to have to move? Initial student models in this unit propose a variety of ideas, but it seems like melting polar ice is a likely cause for this global phenomenon. Uncertainty and student concern for the people impacted motivate unit investigations that help students better understand the matter and energy flows that underlie a global phenomenon like polar ice melt and sea level rise.
Historical data, hands-on investigations, and typical early-year math (like unit conversions) help students establish the mechanisms that cause sea level rise and estimate its potential impact. Through investigations, simulations, and system models, students figure out how decreasing carbon dioxide emissions and two geoengineering solutions (applying glass microbeads to polar ice and protecting glaciers from warm water with berms) could help slow polar ice melt, protecting coastal communities. As they do so, they
1) begin developing the science practices needed in a chemistry classroom,
2) build a particle-level, quantifiable understanding of thermodynamics, and
3) consider how human activity results in particle-level changes with global implications.

Simulations
Unit Examples
Additional Unit Information
Next Generation Science Standards Addressed in this Unit
Performance Expectations

This unit builds toward these performance expectations:
- HS-PS3-1† Create a computational model to calculate the change in the energy of one component in a system when the change in energy of the other component(s) and energy flow in and out of the system are known.
- HS-PS3-4 Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperatures are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).
- HS-ESS2-2 Analyze geoscience data to make the claim that one change to Earth’s surface can create feedback that causes changes to other Earth systems.
- HS-ESS2-4 Use a model to describe how variations in the flow of energy into and out of Earth’s systems result in changes in climate.
- HS-ESS2-7† Construct an argument based on evidence about the simultaneous coevolution of Earth’s systems and life on Earth.
- HS-ESS3-1* Construct an explanation based on evidence for how the availability of natural resources, occurrence of natural hazards, and changes in climate have influenced human activity.
- HS-ESS3-5 Analyze geoscience data and the results from global climate models to make an evidence-based forecast of the current rate of global or regional climate change and associated future impacts on Earth systems.
- HS-ESS3-6† Use a computational representation to illustrate the relationships among Earth systems and how those relationships are being modified due to human activity.
*This performance expectation is developed across multiple units.
†This performance expectation is developed across multiple courses.
Disciplinary Core Ideas

PS3.A: Definitions of Energy
- Energy is a quantitative property of a system that depends on the motion and interactions of matter and radiation within that system. That there is a single quantity called energy is due to the fact that a system’s total energy is conserved, even as, within the system, energy is continually transferred from one object to another
and between its various possible forms. (HS-PS3-1, HS-PS3-2)
PS3.B: Conservation of Energy and Energy Transfer
- Conservation of energy means that the total change of energy in any system is always equal to the total energy transferred into or out of the system. (HS-PS3-1)
- Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems. (HS-PS3-1, HS-PS3-4)
- Mathematical expressions, which quantify how the stored energy in a system depends on
its configuration (e.g. relative positions of charged particles, compression of a spring) and how kinetic energy depends on mass andspeed, allow the concept of conservation of energy to be used to predict and describe system behavior. (HS-PS3-1) - Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g.,
water flows downhill,objects hotter than their surrounding environment cool down). (HS-PS3-4)
PS3.D: Energy in Chemical Processes
- Although energy cannot be destroyed, it can be converted to less useful forms—for example, to thermal energy in the surrounding environment. (HS-PS3-3, HS-PS3-4)
ESS1.B: Earth and the Solar System
- Cyclical changes in the shape of Earth’s orbit around the sun, together with changes in the tilt of the planet’s axis of rotation, both occurring over hundreds of thousands of years, have altered the intensity and distribution of sunlight falling on the earth. These phenomena cause a cycle of ice ages and other gradual climate changes. (secondary to HS-ESS2-4)
ESS2.A: Earth Materials and System
- Earth’s systems, being dynamic and interacting, cause feedback effects that can increase or decrease the original changes. (HS-ESS2-1, HS-ESS2-2)
- The geological record shows that changes to global and regional climate can be caused by interactions among changes in the sun’s energy output or Earth’s orbit, tectonic events, ocean circulation, volcanic activity, glaciers, vegetation, and human activities. These changes can occur on a variety of time scales from sudden (e.g., volcanic ash clouds) to intermediate (ice ages) to very long-term tectonic cycles. (HS-ESS2-4)
ESS2.D: Weather and Climate
- The foundation for Earth’s global climate systems is the electromagnetic radiation from the Sun, as well as its reflection, absorption, storage, and redistribution among the atmosphere, ocean, and land systems, and this energy’s re-radiation into space. (HS-ESS2-4)
- Gradual atmospheric changes were due to plants and other organisms that captured carbon dioxide and released oxygen. (HS-ESS2-6, HS-ESS2-7)
- Changes in the atmosphere due to human activity have increased carbon dioxide concentrations and thus affect climate. (HS-ESS2-4, HS-ESS2-6)
- Current models predict that, although future regional climate changes will be complex and varied, average global temperatures will continue to rise. The outcomes predicted by global climate models strongly depend on the amounts of human-generated greenhouse gases added to the atmosphere each year and by the ways in which these gases are absorbed by the ocean and biosphere. (secondary to HS-ESS3-6)
ESS2.E: Biogeology
- The many dynamic and delicate feedbacks between the biosphere and other Earth systems cause a continual co-evolution of Earth’s surface and the life that exists on it. (HS-ESS2-7)
ESS3.A: Natural Resources
- Resource availability has guided
the development ofhuman society. (HS-ESS3-1)
ESS3.B: Natural Hazards
- Natural hazards and other geologic events have shaped the course of human history; [they] have significantly altered the sizes of human populations and have driven human migrations. (HS-ESS3-1)
ESS3.D: Global Climate Change
- Though the magnitudes of human impacts are greater than they have ever been, so too are human abilities to model, predict, and manage current and future impacts. (HS-ESS3-5)
- Through computer simulations and other studies, important discoveries are still being made about how the ocean, the atmosphere, and the biosphere interact and are modified in response to human activities. (HS-ESS3-6)
Science & Engineering Practices

Asking Questions and Defining Problems: This unit intentionally develops students’ engagement in these practice elements:
- 1.1 Ask questions that arise from careful observation of phenomena, or unexpected results, to clarify and/or seek additional information.
- 1.2 Ask questions that arise from examining models or a theory, to clarify and/or seek additional information and relationships.
- 1.3 Ask questions to determine relationships, including quantitative relationships, between independent and dependent variables.
- 1.4 Ask questions to clarify and refine a model,
an explanation, or an engineering problem. - 1.5 Evaluate a question to determine if it is testable and relevant.
- 1.6 Ask questions that can be investigated within the
scope of theschool laboratory, research facilities, orfield (e.g., outdoor environment) with available resources and, when appropriate, frame a hypothesis based on a model or theory. - 1.7 Ask
and/or evaluatequestions that challenge thepremise(s) of an argument, the interpretation of a data set, or thesuitability of a design.
Planning and Carrying Out Investigations: This unit intentionally develops students’ engagement in these practice elements:
- 3.1 Plan an investigation
or test a design individually andcollaboratively to produce data to serve as the basis for evidence as part ofbuilding andrevising models,supporting explanations for phenomena, or testing solutions to problems. Consider possible confounding variables or effects and evaluate the investigation’s design to ensure variables are controlled. - 3.2 Plan and conduct an investigation individually and collaboratively to produce data to serve as the basis for evidence, and in the design: decide on types, how much, and accuracy of data needed to produce reliable measurements and consider limitations on the precision of the data (e.g., number of trials, cost, risk, time),
and refine the design accordingly. - 3.3 Plan and conduct an investigation or test a design solution in a safe and ethical manner including considerations of
environmental, social, andpersonal impacts. - 3.4 Select appropriate tools to collect,
record, analyze, and evaluatedata. - 3.5 Make directional hypotheses that specify what happens to a dependent variable when an independent variable is manipulated.
Using Mathematics and Computational Thinking: This unit intentionally develops students’ engagement in these practice elements:
- 5.1 Create
and/or revisea computational model or simulation of aphenomenon, designed device, process, orsystem. - 5.2 Use mathematical, computational,
and/or algorithmicrepresentations of phenomena or design solutions todescribe and/orsupport claimsand/or explanations. - 5.3 Apply techniques of algebra and functions to represent
and solvescientificand engineeringproblems. - 5.4 Use simple limit cases to test
mathematical expressions, computer programs, algorithms, orsimulations of a process or system to see if a model “makes sense” by comparing the outcomes with what is known about the real world. - 5.5 Apply ratios,
rates, percentages,and unit conversions in the context of complicated measurement problems involving quantities with derived or compound units (such as mg/mL, kg/m3, acre-feet, etc.).
The following practices are also key to the sensemaking in this unit:
- Developing and Using Models
- Analyzing and Interpreting Data
- Engaging in Argument from Evidence
- Obtaining, Evaluating, and Communicating Information
Crosscutting Concepts

Systems and Systems Models: This unit intentionally develops students’ engagement in these practice elements:
- 4.1 Systems can be designed to do specific tasks.
- 4.2 When investigating or describing a system, the boundaries and initial conditions of the system need to be defined and their inputs and outputs analyzed and described using models.
- 4.3 Models (e.g., physical, mathematical, computer models) can be used to simulate systems and interactions—including energy, matter,
and informationflows—within and between systems at different scales. - 4.4 Models can be used to predict the behavior of a system, but these predictions have limited precision and reliability due to the assumptions and approximations inherent in models.
Energy and Matter: This unit intentionally develops students’ engagement in these practice elements:
- 5.1 The total amount of energy and matter in closed systems is conserved.
- 5.2 Changes of energy and matter in a system can be described in terms of energy and matter flows into, out of, and within that system.
- 5.3 Energy cannot be created or destroyed—only moves between one place and another place, between objects
and/or fields, or between systems. - 5.4 Energy drives the cycling of matter within and between systems.
The following crosscutting concepts are also key to the sensemaking in this unit:
- Stability and Change
- Scale, Proportion, and Quantity
Connections to the Nature of Science

Which elements of the Nature of Science (NOS) are developed in the unit?
- Science investigations use diverse methods and do not always use the same set of procedures to obtain data.
- Scientific inquiry is characterized by a common set of values that include: logical thinking, precision, open-mindedness, objectivity, skepticism, replicability of results, and honest and ethical reporting of findings.
- Science arguments are strengthened by multiple lines of evidence supporting a single explanation
- Most scientific knowledge is quite durable but is, in principle, subject to change based on new evidence and/or reinterpretation of existing evidence.
- Scientists often use hypotheses to develop and test theories and explanations.
- Science is a unique way of knowing and there are other ways of knowing.
- Science and technology may raise ethical issues for which science, by itself, does not provide answers and solutions.
- Many decisions are not made using science alone, but rely on social and cultural contexts to resolve issues.
How are they developed?
- Students engage with a variety of investigations throughout the unit.
- Students are encouraged to be precise in data collection, honest about their results, and appropriately skeptical of ideas.
- Students gather multiple types of evidence (e.g., historical and experimental data, or experimental and simulation-based data) to support their ideas.
- Students read about how scientists change their climate models based on updated assumptions or new data.
- Students develop their own hypotheses based on prior understanding and test these hypotheses.
- Students explicitly compare the ways of knowing of Inuit hunters and fishers and NASA scientists.
- Scientists consider aspects of the Learning in Places Ethical Deliberation and Decision-Making in Socio-Ecological Systems Framework as they consider the implications of potential solutions to polar ice melt.
- Students hear from a variety of perspectives as they consider whether the proposed solutions would have a positive impact.
Unit Placement Information
What is the anchoring phenomenon and why was it chosen?

For the anchoring phenomenon, students explore stories of and data about communities that are impacted by and responding to sea level rise. These responses often involve human migration away from rising sea levels. Students consider where water is found on Earth and develop models about this phenomenon that share initial ideas about how and why the sea level is rising. Because this phenomenon of sea level rise is driven in significant part by polar ice melt, it provides a meaningful context in which students can consider not only anthropogenic climate change and its impacts, but also physical science ideas around energy transfer. This phenomenon also generated high student interest across racial and gender identities and location (rural/urban) in a national survey.
Furthermore, it successfully garnered student interest in the unit field test. Furthermore, this anchoring phenomenon provides a compelling example of the problems caused by human-caused climate change. Students will explicitly return to issues related to climate change in OpenSciEd Unit C.4: Why are oysters dying, and how can we use chemistry to protect them? (Oysters Unit) (ocean acidification), OpenSciEd Unit C.5: Which fuels should we design our next generation vehicles to use? (Fuels Unit) (replacing fossil fuels in transportation), and OpenSciEd Unit P.1: How can we design more reliable systems to meet our communities’ energy needs? (Electricity Unit) (improving the electricity grid). There will also be implicit connections in the remaining chemistry units, OpenSciEd Unit C.2: What causes lightning and why are some places safer than others when it strikes? (Electrostatics Unit) (as students think about the dangers of lightning, as extreme storms become more frequent due to climate change) and OpenSciEd Unit C.3: How could we find and use the resources we need to live beyond Earth? (Space Survival Unit) (as students consider if human beings could live off of Earth).
Where does this unit fall within the OpenSciEd Scope and Sequence?

This unit is the first in the OpenSciEd High School Chemistry course sequence. In OpenSciEd HS Biology, students built proficiency with crosscutting concepts and science and engineering practices that are leveraged throughout this unit. They also began to build understandings of speciation and the role of human activity in Earth systems, which they build on in this unit. Several of the Performance Expectations (PEs) in this unit are shared across other units across OpenSciEd High School courses. HS-PS3-1 is shared with OpenSciEd Unit C.5: Which fuels should we design our next generation vehicles to use? (Fuels Unit), OpenSciEd Unit P.1: How can we design more reliable systems to meet our communities’ energy needs? (Electricity Unit), and OpenSciEd Unit P.4: Meteors, Orbits, and Gravity (Meteors Unit). HS-ESS2-7 is shared with OpenSciEd Unit B.5: How did polar bears evolve and what will happen to them as their environment changes? (Natural Selection Unit). HSESS3-1 is shared with OpenSciEd Unit C.5: Which fuels should we design our next generation vehicles to use? (Fuels Unit). HS-ESS3-6 is shared with OpenSciEd Unit B.2: What causes fires in ecosystems to burn and how should we manage them? (Fires Unit). HS-PS3-4, HS-ESS2-2, HS-ESS2-4, and HS-ESS3-5 are addressed in this unit alone in the OpenSciEd course sequence.
As this unit is intended as the first in the OpenSciEd High School Chemistry course, it intentionally incorporates, in the moment, a number of activities that would typically take place in a “Unit 0.” These include co-construction of Community Agreements, investigation planning, use of some simple laboratory equipment, lab safety measures, measurement, scientific notation, and unit conversions. We recommend you begin this unit at the start of the school year, following any requirements in your school or district. In addition, students may benefit from a collaborative hands-on activity prior to the start of this unit. This will help prepare students to talk with each other, work together, and ask questions about phenomena throughout the unit and course.
How is the unit structured?

The unit is organized into three lesson sets. Lesson Set 1 (Lessons 1-4) focuses on explaining sea level rise with science ideas about how increased energy in Earth’s systems causes polar ice melt. Lesson Set 2 (Lessons 5-7) emphasizes how spreading microbeads on polar ice or reducing carbon dioxide emissions could reduce polar ice melt, and therefore sea level rise, by disrupting harmful feedback loops. This lesson set also introduces a berm as another possible solution to slow sea level rise. Lesson Set 3 (Lessons 8-13) supports students as they figure out how the berm solution and quantify its impact. Students bring these ideas together in a climate model in Lesson 13, then apply them in a transfer task.

What modifications will I need to make if this unit is taught out of sequence?

This is the first unit of the High School Chemistry Course in the OpenSciEd Scope and Sequence. Given this placement, several modifications would need to be made if teaching chemistry first or teaching this unit later in the Chemistry course. These include the following adjustments:
- Early units spend considerable time developing Community Agreements. If taught out of sequence, later units will need additional time for developing the Community Agreements.
- If taught before OpenSciEd High School Biology, supplemental modeling of energy flow within and between systems may be required. Students will also need more explicit callbacks to middle school understandings of evolution as they consider the feedback loops that occur between the biosphere and other spheres over long periods of time.
- If you are using this unit to address the topics for “Unit 9 – Global Change” as organized by College Board in the Course and Exam Description for AP Environmental Science 2020, be prepared to supplement with materials about other greenhouse gases besides carbon dioxide, impacts of climate change besides increased temperature including ocean acidification (as in [material: cc]), changes in oceanic currents, changes in where species of animals live, and explicit consideration of the importance of ozone in the atmosphere.
- Major modifications would be needed to this unit to address the topics for the College Board’s 2022 AP Chemistry “Unit 6 – Thermodynamics” or its 2020 AP Physics 2 “Unit 2 – Thermodynamics” because these courses divide the thermodynamics ideas built in this unit while adding many other ideas. Carefully consult the course and exam descriptions
How do I shorten or condense the unit if needed? How can I extend the unit if needed?

The following are example options to shorten or condense parts of the unit without eliminating important sensemaking for students:
- Lesson 3: Planning the investigation is important, but actually collecting the data is less vital. If tight on time, students may be presented with sample data after planning the investigation. Although a simpler version of this investigation is also included in [material: bo] with a different emphasis, this activity may also go quicker if students have also experienced that unit.
- Lesson 4: Once again, the investigation plan is more important to sensemaking than the actual investigation. If you believe ice melting will have to take place during class, prepare a “before and after” set of samples ahead of time so that students can see the change without having to collect data themselves.
- Lesson 9: Students should conduct the first investigation themselves so they have experience using scales. However, the demonstration may be done with the provided videos instead.
- Lessons 10-11: Students should plan the investigations and need to use the first simulation in Lesson 10, but may use provided data for developing conclusions in one of the lessons if necessary for time purposes.
- Lesson 12: Students’ computations may be reduced or done partially as a class, as long as students have the opportunity to show some calculations and make a claim based on this work.
To extend or enhance the unit, consider the following:
- Lesson 1: Give students opportunities to consider how their own ancestors have migrated in the past and how their experiences might have been similar to or different from those of climate migrants. Guidance is also provided to support students in thinking about whether species that move or spread because of climate change should be labeled by people as “invasive.”
- Lesson 2: Give students time to analyze geological and historical data from your own region in addition to the global data provided in the lesson.
- Lesson 5: Share the provided resource for value mapping with students. This will provide students ideas of what they are looking for in the solutions throughout Lesson Sets 2 and 3.
- Lesson 6: Assign students to research solutions that leverage albedo to decrease the “urban heat island” effect.
- Lesson 9: If students proposed thermal expansion of water as a cause of sea level rise, establish this second correct mechanism and a volume-temperature relationship by placing a capped syringe in warm water and watching it expand, as done in OpenSciEd Unit C.5: Which fuels should we design our next generation vehicles to use? (Fuels Unit). Introduce different types of glassware beyond those included in the lesson, if you believe it is important for students to be familiar with all equipment in the chemistry lab.
- Lesson 10: Encourage students to collect data on the related phenomenon they identify in the lesson. Give students time to graph by hand if you think this will support their understanding of graphs.
- Lesson 11: Help students generalize their understanding of the energy to melt a mass of ice to all substances by assigning and discussing Other Substances.
- Lesson 13: Return to climate models from throughout the course. Have students explore these models in more depth, paying particular attention to the background information accessible in each.
- All lessons: Remove scaffolds provided with Science and Engineering Practices as a way to give students more independent work with the elements of these practices.
Unit Acknowledgements
Unit Development Team

- Dan Voss, Revision Unit Lead, Northwestern University
- Tara McGill, Field Test Unit Lead, Northwestern University
- Michael Novak, Field Test Unit Lead & Sim Developer, Northwestern University
- Melissa Campanella, Writer, University of Colorado Boulder
- Harrison Davis, Lesson Tester, DC Public Schools
- Arlene Friend, Writer, Denver Public Schools
- Jacob Noll, Lesson Tester, Niles North, Niles Township High Schools D219
- Rachel Patton, Development Support, Denver Public Schools
- Samantha Pinter, Writer, Norwalk Public Schools & Northwestern University
- Ann Rivet, Advisor on ESS Integration, Teachers College Columbia University
- Kerri Wingert, Writer & Coherence Reviewer, University of Colorado Boulder
- Michelle Zhang, Writer & Lesson Tester, Oak Park & River Forest HS District 200
Production Team

- Madison Hammer, Production Manager, University of Colorado Boulder
- Erin Howe, Project Manager, University of Colorado Boulder
- Stephanie Roberts, Copy Editor, Beehive Editing
Unit External Evaluation
NextGenScience’s Science Peer Review Panel

An integral component of OpenSciEd’s development process is external validation of alignment to the Next Generation Science Standards by NextGenScience’s Science Peer Review Panel using the EQuIP Rubric for Science. We are proud that this unit has earned the highest score available and has been awarded the NGSS Design Badge. You can find additional information and read this unit’s review on the nextgenscience.org website.
Unit standards
This unit builds toward the following NGSS Performance Expectations (PEs) as described in the OpenSciEd Scope & Sequence:
- HS-PS3-1†
- HS-PS3-4
- HS-ESS2-2
- HS-ESS2-4
- HS-ESS2-7†
- HS-ESS3-1*
- HS-ESS3-5
- HS-ESS3-6†
Reference to kit materials
The OpenSciEd units are designed for hands-on learning and therefore materials are necessary to teach the unit. These materials can be purchased as science kits or assembled using the kit material list.